MBI Videos
James Keener
-
 James Keener
James KeenerDiffusion is the enemy of life. This is because diffusion causes small particles to spread out, and for aggregates of particles to dissipate. Thus, in order to be alive and maintain its structure, an organism must have ways to counteract the constant tendency of things to spread out. And indeed they do. Plants, for example, are able to harness the energy of the sun to convert carbon dioxide and water into high energy compounds such as carbohydrates. These high energy compounds are then carefully deconstructed by living organisms to do work moving things around and building and repairing their structures. In this way, living things are able to combat the tendency of structures to dissipate and fall apart.
However, living organisms do much more than simply counteract diffusion; they actually exploit it for specific purposes. That is, they expend energy to concentrate molecules and then use the fact that molecules move by diffusion down their concentration gradient to do useful things. How do they do this? The short answer is that they couple diffusion with appropriate chemical reactions and are thereby able to exploit the inherent diffusive motion of molecules. Indeed, many of the processes that take place in living cells can be described as the interaction of reacting and diffusing chemical species. This realization has led to the mathematical description of many interesting biological processes and this in turn has led to an increased understanding of how biological systems work.
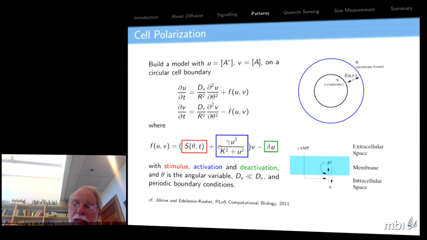
In this talk, I give several examples of the ways that cells use diffusion to their advantage, and describe the equations that model these processes. In particular, I will describe how molecular diffusion and reaction are used to make signals, to create functional aggregates, to take a census, and to make length measurements.
In this way, I hope to convince you that living organisms have made diffusion their friend, not their enemy, and in the process, demonstrate the importance of understanding the solutions of the equations governing diffusion-reaction processes.
-
 James KeenerThere are a number of interesting and important biological processes that are best modelled as two-phase material mixtures. These include mucin exocytosis and transport, blood clot formation and biofilm formation. These all involve the interplay between flow, physical structure, mechanics and chemistry in a environment with complex dynamic geometry. The mathematical description of these processes requires equations describing multiphase flow, the evolution of composition and structure, and the relationship between stresses and composition/ structure (i.e., constitutive relations). Additionally, these equations of motion must properly account for interactions of the complex materials with dynamic physical boundaries, moving interfaces between materials with markedly different physical properties, and typically include strongly nonlinear constitutive relations or rate expressions.
James KeenerThere are a number of interesting and important biological processes that are best modelled as two-phase material mixtures. These include mucin exocytosis and transport, blood clot formation and biofilm formation. These all involve the interplay between flow, physical structure, mechanics and chemistry in a environment with complex dynamic geometry. The mathematical description of these processes requires equations describing multiphase flow, the evolution of composition and structure, and the relationship between stresses and composition/ structure (i.e., constitutive relations). Additionally, these equations of motion must properly account for interactions of the complex materials with dynamic physical boundaries, moving interfaces between materials with markedly different physical properties, and typically include strongly nonlinear constitutive relations or rate expressions.
In this talk, I will describe two features of mucus: the dynamics of mucus vesicle exocytosis and its transport of acid against an acid gradient.
The short story is as follows: Mucin is packaged into vesicles at very high concentration (volume fraction) and when the vesicle is released to the extracellular environment, the mucin expands in volume by two orders of magnitude in a matter of seconds. This rapid expansion is mediated by the rapid exchange of calcium and sodium that changes the crosslinking structure of the mucin polymers, thereby causing it to swell. I will describe a model of gel swelling and deswelling that accounts for these features, and is an interesting free boundary problem.
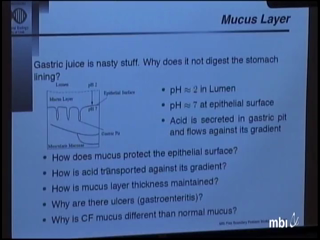
One of the important functions of the mucus lining of the stomach is to allow digestion of food to take place without the lining of the stomach being digested. An intriguing question is how acid can be released into the lumen of the stomach while maintaining a low concentration of hydrogen ions near the epithelial lining. A possible answer is that the flow of acid against its gradient is mediated by buffering by mucin. When mucin is secreted it rapidly binds hydrogen, but when it reaches the lumen where the pH is low, mucin is degraded by pH-activated pepsin, releasing its acid. The model associated with this process includes a free boundary problem to determine the thickness of the mucus layer and its acid-protective ability. -
 James KeenerNo abstract provided.
James KeenerNo abstract provided.
